In which region is the substance in both the solid phase and the liquid phase?
1 2 3 4
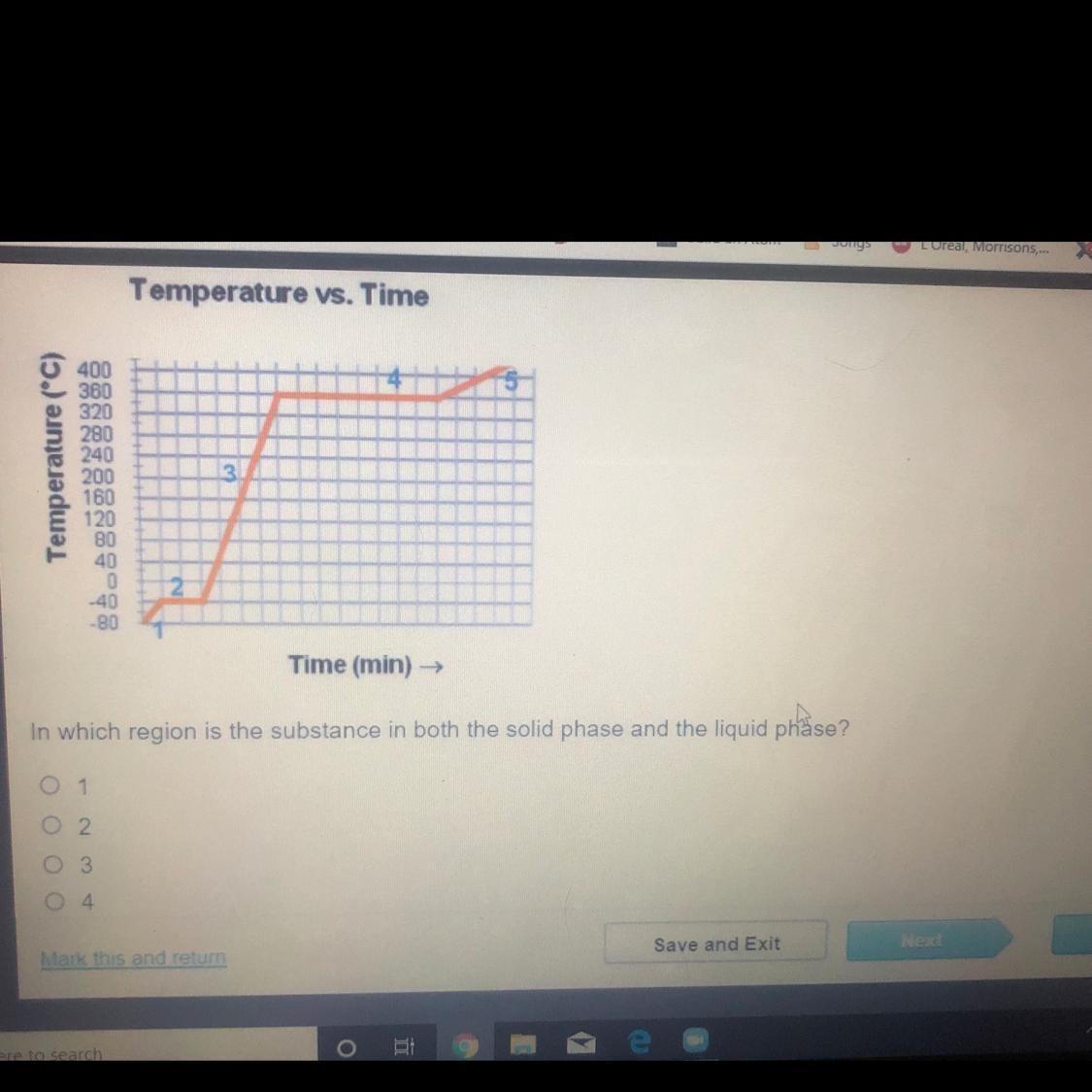
Answers
Answer:
its 2 on ed I promise do not listen to the brainiest
Explanation:
Answer:
B)2
Explanation:
Related Questions
Which components are part of all scientific investigations? Check all that apply.
Answers
Answer:
Steps of a scientific investigation include 1. make observation
2. ask a question
3. form a hypothesis
4. test the hypothesis
5. analyze results
5 , drawing conclusions,
6. and communicating the results
What is the atomic number of an atom?
O the number of neutrons
O the number of electrons and neutrons
O the number of protons
the number protons and neutrons
Answers
Changes in the number of neutrons will change the individual isotope of that element that the atom is, but the atomic number is always equal to the number of protons.
Also, protons + neutrons = mass number, not atomic number!
Which tissues and organs belong to the Musculoskeletal System?
Answers
What’s this called
Please ASAP
Answers
Answer:
carbon dioxide CO[tex]{2}[/tex]
Explanation:
Double Polar Covalent bonds with CO2
Explanation:
Keep in mind that CO2 is a nonpolar molecule
Which phrase describes density?
O height of a point above sea level
O force pushing on an area or a surface
O amount of mass in a certain volume
O amount of matter an object contains
Answers
Answer:
C: amount of mass in a certain volume
Explanation:
Just took the quiz and got 100%
Help plzzzzz ASAP!!!!!!!
Answers
Answer:
3. The total number of protons and neutrons in an atom is its mass number.
4. The mass number of a helium atom with 2 protons and 2 neutrons is 4.
5. A beryllium atom with 4 protons and a mass number of 9 has 5 neutrons.
6. The chemical symbol is Ne. The atomic number is 10. The mass number is 22.
7. Neon-22
8. Not enough information
If 23,000 joules of energy are used to heat mercury by 4.00 °C, what is the mass of the mercury?
Answers
Match the element with their place on the periodic table.
Question 4 options:
Period 4, group 5-
Period 5, group 7-
Period 7, group 5-
Period 5, group 4-
1. dubrium
2. technetium
3. zirconium
4. vanadium
Answers
Answer:
Period 4, group 5- Vanadium
Period 5, group 7- Technetium
Period 7, group 5- Dubnium
Period 5, group 4- Zirconium
Explanation:
What information does a subscript in a chemical formula provide? Choose all that apply. * The charge of that atom while in the compound How many of each atom are present in the compound How many valence electrons each element has while in the compound How many valence electrons each element starts with In ionic, the simplified ratio of atoms in relation to each other
Answers
Answer:
B. How many of each atom are present in the compound
D. the simplified ratio of atoms in relation to each other
Explanation:
In a chemical formula, chemical elements or atoms are represented by a chemical symbol for example Fe for iron and Na for sodium, and the number of each atom is represented by a subscript such as CO2, where 2 is a subscript representing 2 atoms of oxygen.
A subscript represents the number of each atom in the compound and the simplified ratio of atoms in relation to each other. The simplified ratio of atoms in relation to each other means subscript shows the contribution of both the atoms in the compound, for example: N2 + 3H2 => 2NH3, it means the subscript showing the ratio or proportionate of atoms that is 2:2 for both nitrogen and hydrogen.
The subscript is always written below and to the right of the chemical symbol.
Hence, the correct answer is "B. How many of each atom are present in the compound and D. the simplified ratio of atoms in relation to each other"
Un compuesto formado por carbono, hidrógeno y oxígeno tiene una masa de 4,6 g. Se hace reaccionar con 9,6 g de oxígeno dando 8,8 g de CO2 y 5,4 g de agua. Si cogemos 9,2 g de un compuesto en un volumen 5,80l en P= 780 mmHg a una temperatura de 90ºC. Calcula la fórmula empírica y molecular.
Answers
Answer:
La fórmula empírica y molecular es: C₂H₆O.
Explanation:
Para calcular la formula empírica y molecular del compuesto debemos primero plantear la reacción:
[tex] C_{x}H_{y}O_{z} + O_{2} \rightarrow CO_{2} + H_{2}O [/tex]
Necesitamos encontrar "x", "y" y "z". Para ello, tenemos que recordar que la masa de carbono e hidrógeno producida está relacionada con la cantidad de C y H inicial (del compuesto):
Para el H:
CHO → H₂O
y 5,4g
[tex] \frac{2*1 g}{18 g} = \frac{y}{5,4 g} \rightarrow y = 0,6 g [/tex]
Para C:
CHO → CO₂
x 8,8g
[tex] \frac{12 g}{44 g} = \frac{x}{8,8 g} \rightarrow x = 2,4 g [/tex]
Para el O:
[tex] z = 4,6 g - 2,4 g - 0,6 g = 1,6 g [/tex]
Ahora mediante el calculo de los moles del C, H y O podemos encontrar la fórmula empírica:
Para el H:
[tex] n_{y} = \frac{m}{Pm} = \frac{0,6 g}{1 g/mol} = 0,6 moles [/tex]
Para el C:
[tex] n_{x} = \frac{m}{Pm} = \frac{2,4 g}{12 g/mol} = 0,2 moles [/tex]
Para el O:
[tex] n_{z} = \frac{m}{Pm} = \frac{1,6 g}{16 g/mol} = 0,1 moles [/tex]
[tex] C_{\frac{n_{x}}{n_{z}}}H_{\frac{n_{y}}{n_{z}}}O_{\frac{n_{z}}{n_{z}}} = C_{\frac{0,2}{0,1}}H_{\frac{0,6}{0,1}}O_{\frac{0,1}{0,1}} = C_{2}H_{6}O_{1} [/tex]
Entonces, la fórmula empírica del commpuesto formado es C₂H₆O.
Ahora para determinar la fórmula molecular podemos usar la siguiente relación:
[tex] \frac{Pm}{Pm_{e}} = n [/tex]
[tex] F_{m} = n*F_{e} [/tex]
[tex] F_{m} = \frac{Pm}{Pm_{e}}*F_{e} [/tex]
En donde Fm (fórmula molecular) y Fe (fórmula empírica) están relacionadas por n.
El valor de Pm lo obtenemos de la ecuación del gas ideal:
[tex]PV = nRT = \frac{m}{Pm}RT[/tex]
[tex] Pm = \frac{mRT}{PV} = \frac{9,2 g*0,082 L*atm/(K*mol)*(90 + 273 K)}{1.02 atm*5,80 L} = 46,3 g/mol [/tex]
[tex] F_{m} = \frac{46,3 g/mol}{(2*12 + 6*1 + 16)g/mol}*C_{2}H_{6}O_{1} = 1.00*C_{2}H_{6}O_{1} = C_{2}H_{6}O_{1} [/tex]
Por lo tanto, la fórmula molecular es la misma que la fórmula empírica, a saber C₂H₆O.
Espero que te sea de utilidad!
Which scientist did people think was insane so they dismissed his ideas for almost 2000 years?
Answers
I think let me know if I am wrong
Learning Task No. 2: Using the Periodic Table of Elements, determine the ele-
ments asked in each guide question. Write your answer in your notebook.
Guide Questions:
1. Which elements are likely to lose electrons?
2. Which elements are likely to ain electrons?
3. Which type of elements are likely to have no electrical charge at all?
Answers
Explanation:
1 metal they are likely to lose an electron
2non metals
3 Nobel gases because they are not reactive with stable valance number
i dont get what it means by of touch
Answers
Answer:
So I don't know what do you mean by that, please be a little specific.
Explanation:
^^
Hydrogen atoms are excited by a laser to the =4 state and then allowed to emit.
What is the maximum number of distinct emission spectral lines (lines of different wavelengths) that can be observed from this system? Calculate the wavelength of the 4⟶3 transition.
Answers
Answer:
1875 nm
Explanation:
Given the Rydberg formula for hydrogen: 1/λ = R(1/n₁² - 1/n₂²)
where R ≈ 1.097* 10^7 /m
Hence;
1/λ = 1.097* 107/m * (1/3² - 1/4²) = 5.3 * 10^5/m
λ = 1.875 * 10-6 m = 1875 nm
How does an object's mechanical energy change as its speed (velocity) increases?
Answers
Answer:
The mechanical energy of the system increases, provided there is no loss of energy due to friction. The energy would transform to kinetic energy when the speed is increasing. The mechanical energy of the system remains constant provided there is no loss of energy due to friction.
Explanation:
Which of the following is NOT an example of a chemical change? *
O Corrosion
O Grinding
O Combustion
O Rust
Answers
For an object that has a volume of 24.5 mL and a mass of 102g, calculate the density of the object
Answers
Answer:
The answer is 4.16 g/mLExplanation:
The density of a substance can be found by using the formula
[tex]density = \frac{mass}{volume} \\ [/tex]
From the question
mass = 102 g
volume = 24.5 mL
We have
[tex]density = \frac{102}{24.5} \\ = 4.163265306...[/tex]
We have the final answer as
4.16 g/mLHope this helps you
the ability for a substance to rust is
a. oxidation
b.rustability
c.magnetic attraction
d.reactivity
Answers
Answer:
i think it is letter b. rustability but not so sureeee
what part of the scientific method involves drawing inferences about experimental results and determining if the collected data support the original hypothesis?
Answers
Answer:
Drawing Conclusions
Explanation:
You put together your observations and can finally decide if your hypothesis was right or not.
in atmosphere water is solute give reason
Answers
Answer:
water vapour is there
Explanation:
thats all i know
What is the volume of 25.3 g of silver if the density of silver is 10.5 g/mL?
Answers
Answer:
MARK AS BRAINLIST
Explanation:
density = mass/volume
mass=25.3
density =10.5
10.5=25.3/v
v=25.3/10.5
=2.40
combined aggregation of minerals
Answers
Answer:
Rocks
Explanation:
The combined aggregation of minerals are called rocks. There are three types of rocks Igneous Rocks, sedimentary rock, and metamorphic rocks.
Rocks are formed due to the repetitive, orderly, geometrical internal arrangement of minerals. Minerals undergo several processes such as weathering, crystallization, erosion, and sedimentation to form rocks.
Hence, the combined aggregation of minerals is called rocks.
A metal object has a density of 2 g/ml. When placed in a graduated cylinder with 500 ml of water, the
water rises to 508 ml. What is the mass of the metal object?
Answers
Answer:
The answer is 16 gExplanation:
The mass of a substance when given the density and volume can be found by using the formula
mass = Density × volumeFrom the question
density = 2 g/ml
volume = final volume of water - initial volume of water
volume = 508 - 500 = 8 mL
We have
mass = 2 × 8
We have the final answer as
16 gHope this helps you
Which of the following compounds below would possess it trigonal planar molecule or geometry?
PHOTO ABOVE
HELP ME IN MY TEST
Answers
Answer:
B - BF3
Explanation:
BF3 has 3 bonds around the central atom and now lone pairs on it. This matches the geometry of a trigonal planar molecule as per the VSEPR Model.
Molecules have two distinct characteristics: form and geometry. Geometry includes the lone pair encircling the focal element whereas shape excludes it. Therefore, the correct option is option B.
What is VSEPR theory?Valence shell electron repulsive force is what VSEPR stands for. Based on the valence electron pairs that are prevalent around the core element of the molecule, the VSEPR theory is employed to predict the structure and geometry of molecules.
According to VSEPR theory, Lone pair lone pair repulsion is greater than bond pair bond pair repulsion. The core atom of BF[tex]_3[/tex] is surrounded by three bonds and contains no lone pairs. According to the VSEPR Model, this corresponds to the geometry of such a trigonal planar molecule.
Therefore, the correct option is option B.
To know more about VSEPR theory, here:
https://brainly.com/question/19582124
#SPJ6
What volume will 250. mL of gas at STP occupy ig the pressure changes to 2.0 atmospheres and the temperature changes at 30 degrees Celsius
Answers
Answer:
it is 75 degrees
Explanation:
Volume is 138.7 mL.
The equation for calculation of volume is as follows:-
[tex]\frac{P_1V_1}{T_1} =\frac{P_2V_2}{T_2}[/tex]
STP:-
Temperature=273 K
Pressure=1 atm
[tex]\frac{1 atm\times250mL}{273 K} =\frac{2 atm\times V_2}{303\ K}\\V_2=138.7\ mL[/tex]
Hence, the volume is 138.7 mL.
To know more about:-
https://brainly.com/question/15988929
How do we balance Zn + HNO3 Zn(NO3)2 + NO + H20
Answers
3Zn + 8HNO₃⇒ 3Zn(NO₃)₂ + 2NO + 4H₂O
Further explanationEqualization of chemical reaction equations can be done using variables. Steps in equalizing the reaction equation:
1. gives a coefficient on substances involved in the equation of reaction such as a, b, or c etc. 2. make an equation based on the similarity of the number of atoms where the number of atoms = coefficient × index between reactant and product 3. Select the coefficient of the substance with the most complex chemical formula equal to 1For gas combustion reaction which is a reaction of hydrocarbons with oxygen produces CO₂ and H₂O (water vapor). can use steps:
Balancing C atoms, H and the last O atoms
Reaction
Zn + HNO₃⇒ Zn(NO₃)₂ + NO + H₂O
1. gives a coefficientaZn + bHNO₃⇒ Zn(NO₃)₂ + cNO + dH₂O
2. make an equationZn : left = a, right =1 ⇒a=1
H : left = b, right = 2d⇒ b=2d (eq 1)
N : left = b, right = 2+c⇒b=2+c (eq 2)
O : left = 3b, right = 6+c+d ⇒3b=6+c+d(eq 3)
From eq 1 and eq 33(2d)=6+c+d
6d=6+c+d
5d=6+c (eq 4)
From eq 2 and eq 33(2+c)=6+c+d
6+3c=6+c+d
2c=d (eq 5)
From eq 4 and eq 55(2c)=6+c
10c=6+c
9c=6
c = 2/3
input eq 5
d = 2 x 2/3
d = 4/3
input eq 1
b = 2 x 4/3
b = 8/3
The equation
aZn + bHNO₃⇒ Zn(NO₃)₂ + cNO + dH₂O to
Zn + 8/3HNO₃⇒ Zn(NO₃)₂ + 2/3NO + 4/3H₂O x 3
3Zn + 8HNO₃⇒ 3Zn(NO₃)₂ + 2NO + 4H₂O
Based on the Phase Change Graph below, what is the Melting Point for this point
substance?
Answers
Answer:
Where is the phase melting graph? Can't seem to find it
a boy walking in the street potential or kenetic energy?
Answers
because the boy is walking in the street soo it has force to use
Answer:
[tex]\boxed {\tt Kinetic \ energy}[/tex]
Explanation:
First, let's define kinetic energy and potential energy.
Kinetic
energy an object has due to motionPotential
energy an object has stored due to its position, charge, arrangement or other factorsSince the boy is walking down the street, the boy is in motion. Therefore, the energy must be kinetic energy.
Radon-222 is an alpha emitter with a half life of 3.82 days.
1. What is the mass number of the daughter isotope?
2. What is the element symbol of the daughter isotope?
3. What is the atomic number of the daughter isotope?
Answers
The daughter isotope (a decay product): Polonium (Po)
Further explanationRadioactivity is the process of unstable isotopes to stable isotopes by decay, by emitting certain particles,
alpha α particles ₂He⁴ beta β ₋₁e⁰ particles gamma particles γ positron particles ₁e⁰
Radon-222 emits alpha α particles ₂He⁴ , so the atomic number decreases by 2, mass number decreases by 4
Reaction
[tex]\tt _{86}^{222}Rn\Rightarrow _{84}^{218}+_2^4He[/tex]
1. The mass number of the daughter isotope = 218
2 and 3. If we look at the periodic system, the element with atomic number 84 is Polonium (Po)
Suppose the moon orbited the earth just half as fast as it does now.
How many high tides would any one area have in one day?
A-3
B-2
C-1
D-4
Answers
-2
Answer:
Explanation: The correct answer is 1. Do not put 2 .